Background: B-cell receptor (BCR) signals are essential determinants of survival and proliferation throughout normal B-cell development. In B-cell malignancies, these signals are frequently generated by oncogenic mimics of the BCR signaling pathway. For instance, oncogenes in B-ALL, derived from B-cell precursors, typically mimic survival signals from a constitutively active pre-BCR, while tonic and chronic active BCR signaling were identified in mature B-cell lymphomas. Oncogenic BCR-signaling represents an important target of therapeutic intervention: for instance, small molecule inhibitors of SYK (e.g. entospletinib) and BTK (e.g. ibrutinib) have been developed to disrupt oncogenic BCR signaling in mature B-cell lymphomas. Besides SYK and BTK tyrosine kinases, oncogenic BCR-signaling leads to activation of PLCG2, which initiates the release of Ca 2+ from the ER into the cytoplasm. Thereby, calcium flux is decoded by NFATC1 or NF-kB based on fast (NFATC1) or slow (NF-kB) frequencies of Ca 2+-signals.
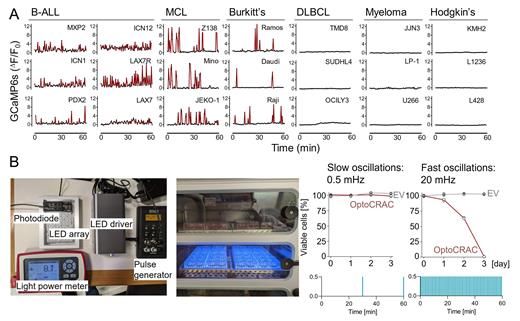
Significance: To interrogate Ca 2+-signals as critical integration point of oncogenic BCR-signaling, we engineered cell lines and PDX with a GCaMP6s-biosensor, which allows tracing of calcium signaling in single cells over time ( Figure A). To cover B-cell malignancies from multiple stages of B-cell development, we engineered B-ALL (pro- and pre-B cells), mantle cell lymphoma (MCL, naïve B-cells), Burkitt's lymphoma (germinal center), DLBCL (post-GC), multiple myeloma (terminally differentiated plasma cells) and Hodgkin's disease (“crippled” BCR-deficient GC-B cells). Strikingly, B-cell malignancies exhibit autonomous Ca 2+-oscillations, which decreased in their frequency from 20 mHz (pro- and pre-B), 11 mHz (naïve), 4 mHz (germinal center) to 0 mHz in post-GC and terminally differentiated plasma cells based on the differentiation stage of their cell of origin ( Figure A).
Results: Given the striking differences in oncogenic BCR-signaling as measured by autonomous Ca 2+ oscillations, we developed an optogenetic system to control the frequency and amplitude of Ca 2+ oscillations by blue light pulses ( Figure B). To model frequency-modulated Ca 2+ signaling in B-cells, we engineered murine pre-B cells carrying an optogenetic tool termed OptoCRAC, which enables reversible activation and inactivation of the plasma membrane Ca 2+ channel Orai1 by intermittent blue light irradiation. Time-lapse imaging of a fluorescent Ca 2+ reporter (R-CaMP1.07) confirmed rapid and reversible Ca 2+ influx in response to intermittent blue light pulses. To assess the phenotypic consequence of high-frequency Ca 2+ oscillations, we developed an LED-array-based optogenetic platform for cell culture plates. While low-frequency Ca 2+ oscillations (0.5 mHz) did not impact cell viability, high-frequency Ca 2+ oscillations (20 mHz) rapidly induced NFAT activation (5 min), and eventually cell death within 72 h ( Figure B).
Mechanism and conclusions: To elucidate the mechanistic contribution of NFATC1 in B-cell death induced by fast Ca 2+ oscillations, we tested whether genetic deletion of Nfatc1 is sufficient to rescue B-cell death upon fast Ca 2+ oscillation induced by 20 mHz intermittent blue light pulses. As expected, while B-ALL cells retaining intact Nfatc1 rapidly decreased cell viability, the cells with Cre-mediated deletion persistently remained in cell culture upon 20 mHz Ca 2+ oscillation. In addition to genetic deletion of Nfatc1, we also found that inducible expression of mutant activators of NF-kB signaling were able to rescue B-cells from cell death induced by high-frequency oscillations: Concurrent expression of Card11 L232LI, MYD88 L265P and IKK2 S177E/S181E not only reduced the frequency of autonomous Ca 2+ oscillations but also enabled B-cell survival despite delivery of light-pulses and Ca 2+ oscillations at a fast 20 mHz frequency. We conclude that autonomous Ca 2+ oscillations represent a critical integration point of oncogenic BCR-signaling in B-cell malignancies and that optogenetic control of BCR-downstream Ca 2+ oscillations will reveal previously unrecognized vulnerabilities that can be exploited to increase efficacy of BCR-signaling inhibitors (e.g. ibrutinib, entospletinib, idelalisib) that are currently being used for the treatment of B-cell lymphomas.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal